


Rheumatology nursing staff received briefings from scores of pharmaceutical giants, including the company which invented the world's best-selling biologic drug – the powerful medication that was wrongly prescribed to hundreds of patients, Express can reveal today.
Published at the start of the year, the Royal College of Physicians' long-awaited review of Jersey's rheumatology department "found the standard of care to be well below what the review team would consider acceptable for a contemporary rheumatological service".
An internal audit of the records of 341 rheumatology patient prescribed biologic drugs last year resulted in approximately one-in-four patients having their biologic drugs discontinued because they were not felt to be necessary.
Pictured: The Royal College of Physicians found that "a recurring theme was the lack of governance, not just in rheumatology but across the healthcare organisation".
The external reviewers also raised concerns that staff may have received training that was "likely to be heavily biased".
Today, Express can reveal that 14 pharmaceutical companies have briefed or trained rheumatology nursing staff over the past decade, while overall Health spending on biologics over the past five years has escalated...
Biologics are a group of powerful drugs derived from natural sources such as human, animal, fungal or microbial cells.
In rheumatology patients, these drugs work by suppressing the immune system and disrupting the inflammation process that leads to joint pain.
Although they tend to work more quickly than traditional rheumatology medication, biologic drugs are considered more potent and can cause some side effects – such as increased risk of infection.
These high-cost drugs are currently the largest expense in the NHS medicines budget, racking up a bill of over £10,000 a year per patient.
Data provided to Express following a request under the Freedom of Information Law showed that the overall Health spend on biologics grew year-on-year between 2019 and 2022 – with a significant jump from £3.9m to £4.9m in 2021.
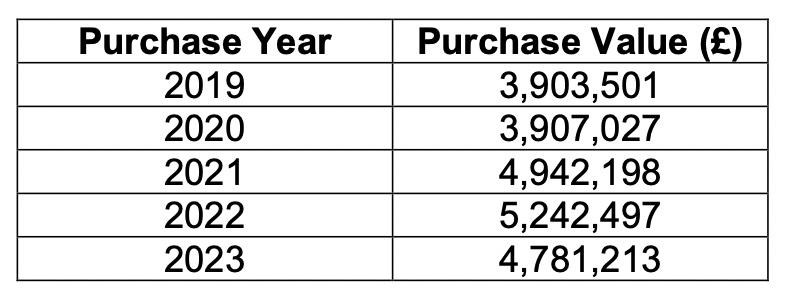
Pictured: The Health Department's annual spend on biologic drugs.
The yearly spend peaked at £5.2m in 2022, before coming down slightly to £4.8m last year.
The data provided did not show how much of this was due to rheumatology patient prescriptions alone.
One of the key concerns raised by the Royal College of Physicians review team was about the Health department's relationship with the pharmaceutical industry in providing training – and potential conflict of interests arising from these arrangements.
The report said: "The review team were concerned to learn that some of the training for specialist [rheumatology] nurses was being provided by the pharmaceutical industry.
"The review team were of the view that the reliance on pharmaceutical companies for drug information and training should be discouraged as it was likely to be heavily biased and that opportunities for both personal and professional development should be embedded within the departmental timetable.”

Pictured: The external reviewers raised concerns that rheumatology nursing staff may have received training that was "likely to be heavily biased".
The Royal College of Physicians recommended that the Health department should “discourage the sole reliance on pharmaceutical companies for drug information and training as it is likely to be heavily biased”.
Express can now reveal that many of the pharmaceutical companies who provided training to local rheumatology nursing staff were the among the world's biggest producers of biologic medicines.
The full list of companies which provided drug information or training to rheumatology nursing staff over the last 10 years is:
AbbVie
Alcura
Apodi
Biogen
GLPG
Healthcare at Home
Johnson & Johnson
Lilly
Novartis
Pfizer
Roche
Sandoz
Sanofi
UCB
Whilst many of this companies are top producers of biologic drugs, AbbVie is arguably the most significant name on this list.
AbbVie's biologic drug HUMIRA is not only the best-selling biologic – it's one of the best-selling drugs worldwide, regardless of class.
Sold under the brand name HUMIRA, adalimumab is a disease-modifying anti-rheumatic drug (DMARD) and monoclonal antibody primarily used to treat rheumatoid arthritis.
The brand name HUMIRA stands for "human monoclonal antibody in rheumatoid arthritis", and was named by one of AbbVie's employees, Richard J. Karwoski, who was also responsible for leading the effort to get HUMIRA approved by the US Food and Drug Administration.
Adalimumab became the first fully human monoclonal antibody approved by the the FDA in 2002.
Sold under brand names HUMIRA and Trudexa, it was approved for use in the European Union one year later.
The drug was first discovered as a result of a collaboration between BASF Bioresearch Corporation and Cambridge Antibody Technology.
HUMIRA was then further manufactured at BASF Bioresearch Corporation, developed by BASF Pharma, and ultimately manufactured and marketed by Abbott Laboratories after Abbott's acquisition of BASF Pharma.
In 2013, Abbott split into two companies – one retaining the Abbott name and the other named AbbVie.
As a result, AbbVie took over development and marketing of HUMIRA.

Pictured: AbbVie's biologic drug HUMIRA is not only the best-selling biologic – it's one of the best-selling drugs worldwide, regardless of class.
Today, AbbVie advertises HUMIRA to help stop irreversible joint damage and improve joint pain, swelling, and stiffness in patients with moderate to severe rheumatoid arthritis.
It can also be used to treat juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn's disease, ulcerative colitis, plaque psoriasis, hidradenitis suppurativa, and uveitis.
HUMIRA is administered by an injection under the skin.
In 2022, the biologic generated nearly $22billion revenue worldwide for AbbVie.
This list was revealed in response to a request submitted by Express under the Freedom of Information Law which asked the Government to confirmed which pharmaceutical companies had provided drug information or training specifically to rheumatology staff in the last ten years.
As part of the same request, Express also asked which pharmaceutical companies had provided drug information or training to any Health staff in the last ten years – but Government could not provide a response as "there is no central record of the information requested".
While many islanders have been taken off biologic drugs since the rheumatology review, the Health Department's relationship with the pharmaceutical industry is a matter of continuing discussion and concern for the HCS Advisory Board.
During a recent meeting, board member Carolyn Downs suggested that the audit of prescribing biologics should be broadened to include the connection between pharmaceutical companies, pharmacists and doctors across the Health department.
Interim Chief Officer Chris Bown reassured the board that the Health department maintains a conflict of interest register.
He said that the executive teams are required to declare any conflicts, and all senior meetings begin with any declarations of conflicts.
Pictured: Interim Chief Officer of Health, Chris Bown with the Royal College of Physicians' damning review of Jersey's rheumatology department.
Board Secretary Emma O’Connor added that all relevant staff are required to complete a form to declare conflicts of interest, and said that the central register will be reviewed monthly to ensure that all declared conflicts are managed appropriately.
Medical Director Patrick Armstrong explained that there is clear guidance on the relationship between staff and pharmaceutical companies in that they should not be directly contacting clinicians directly and staff must not accept direct approaches.
Non-Executive Director Dame Clare Gerada advised that the board is seeking assurance that these guidelines are adhered to.
The Health Department recently announced that a new Immunotherapy Lead Pharmacist – otherwise known as a ‘Biologic Pharmacist’ – has been employed.
Sarah-Jane Stead will be responsible for supporting patients in Jersey with the appropriate use of biologic drugs, including prescribing.
We are delighted to announce that Sarah-Jane Stead has started in the post of Lead Pharmacist, Immunotherapy – otherwise known as a ‘Biologic Pharmacist’.
— Jersey Gov Health & Community Services (HCS) (@GovJsyHCS) February 15, 2024
Sarah-Jane will be responsible for supporting patients with the appropriate use of biologic drugs, including prescribing. pic.twitter.com/LcuRNQTtxn
Chris Bown, Interim Chief Officer for Health and Community Services, said: “HCS is continuing to make good progress on the improvements recommended by the RCP and to ensure that we become a beacon of good governance, not just in rheumatology but across the full spectrum of our health and care services.
"The recruitment of an Immunotherapy Lead Pharmacist was started prior to the RCP review being finalised and aligns with some of recommendations they made."
He added: “Sarah-Jane will be responsible for supporting patients with appropriate use of biologic drugs (including prescribing those drugs).
"She will clinically review and monitor patients’ medication, and ensure compliance with appropriate standards and guidance – providing a key role in clinical audit and governance for biologic medicines.”
New pharmacist to oversee prescription of drugs at centre of critical review
Gov refuses to explain why rheumatology patient list was changed
More than 110 rheumatology patients join potential class action lawsuit
INSIGHT: Why hundreds of Jersey patients were given the wrong drugs
£1.3m funding set aside to deal with 'rheumatology incident'
Health facing class action lawsuit over "inappropriate" prescribing
Comments
Comments on this story express the views of the commentator only, not Bailiwick Publishing. We are unable to guarantee the accuracy of any of those comments.